Results
DROPCELL technology has been reported in various publications and patented by BIOASTER.
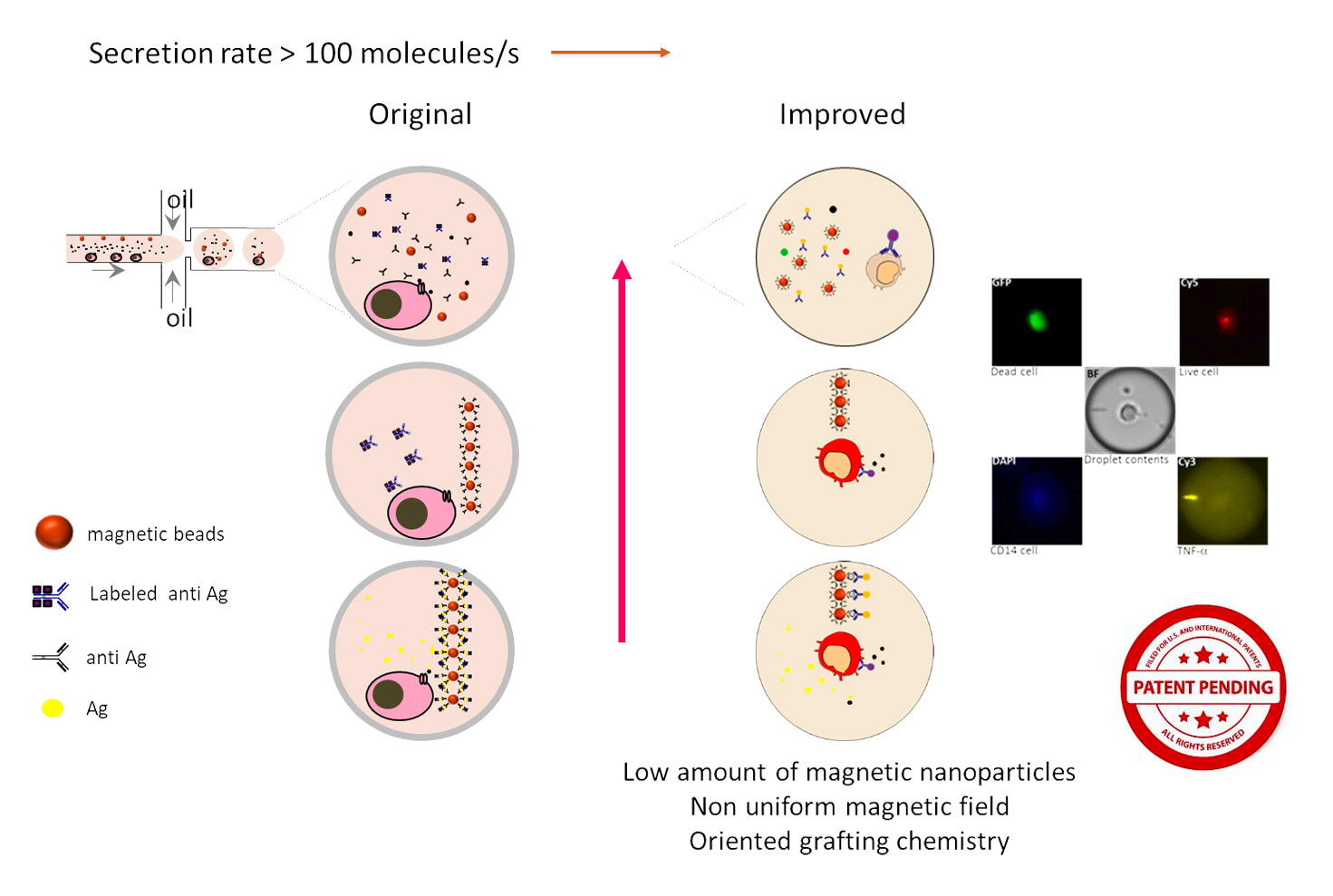
The reported application focuses on the study of TNFa (tumor necrosis factor) secreting profile of monocytes when exposed to Lipopolysaccharides (LPS). A key feature of the droplet-based immunoassay lies in the use of paramagnetic nanoparticles where capture anti-TNFa antibodies are immobilized. Briefly, monocytes are encapsulated together with LPS, anti-TNFa-magnetic nanoparticles and a fluorescent detection antibody. Droplets are placed in an observation chamber specially designed so that droplets form a monolayer. The observation chamber contains a magnet that induces the aggregation of nanoparticles forming a beadline that serves to concentrate the captured analyte and as TNFa secretion progresses, immune-sandwiches form on this beadline, causing the relocation of fluorescence. The fluorescence intensity of beadlines can be determined with the help of rapid image analysis protocols developed by BIOASTER to achieve quantitative assays. The incorporation of magnetic nanoparticles provides a significant advantage by enhancing the sensitivity compared to a magnet-less assay. The study also includes cell viability assessments using fluorescent probes.
DROPCELL technology was successfully applied to clinical samples (healthy donors and septic shock patients).
Advantages
Single-cell droplet microfluidics present several advantages compared to other similar technologies, including:
- High Throughput and parallelization: The Dropcell system can analyze up to 300 000 droplets simultaneously and different conditions can be studied in parallel by using separate observation chambers.
- Cellular compartmentalization: permits the detection of secreted markers providing a more detailed understanding of cell behavior.
- Reduced sample and reagent consumption.
- Increased assay sensitivity.
- Versatility: the size and contents of the droplet can be tailored to each specific application.
Furthermore, the magnetic nanoparticle-based immunoassay developed by BIOASTER, coupled with image analysis protocols offers the capability of monitoring cell responses in real-time and quantitative measurement of secreted molecules.
Overall, the technology allows a fine characterization of a cell population which implies a fine characterization of specific pathologies that can pave the way for applications in diagnostics, prognosis or development of new treatments.
Potential Applications
DROPCELL technology provides first and foremost a platform for the comprehensive characterization of cell populations which can be applied to (1) the progress of diagnostics and discovery of biomarkers, (2) the development of new treatments or vaccines by investigating promising therapeutic targets, (3) high-throughput drug screening, (4) deep understanding of specific pathways and pathologies.
Furthermore, the large datasets generated by single-cell experiments can be adapted for training sets for deep learning or artificial intelligence strategies, allowing the development of in silico models and simulations that mimic cell responses to various stimuli such as vaccines or drugs. Such strategies could reduce or replace animal experiments.
Outlook
The DROPCELL platform is well established in BIOASTER and is used in routine for different internal projects that aim to better characterize cellular responses.
Optimizations were performed to improve the functionalization of magnetic nanoparticles for antibody immobilization. The results of these studies have been recently published in the journal Sensors and Actuators.